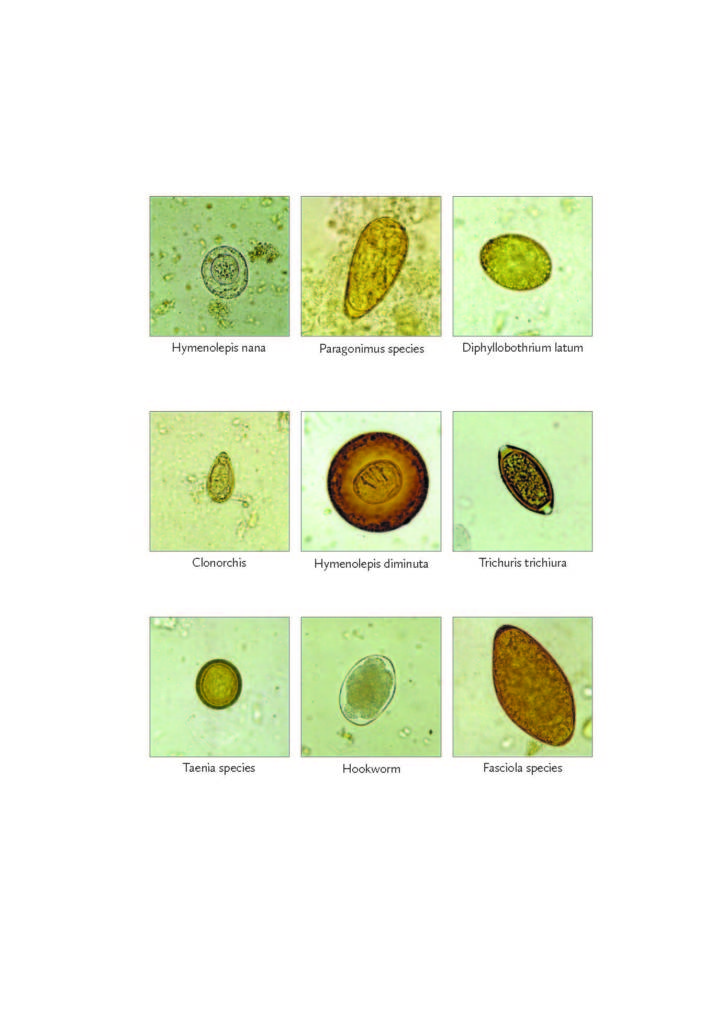
Parasitology
Fixatives, Reagents and Stains
Parasitology Fixatives
10% Formalin Fixative in water
This preservative is a good overall fixative and will fix both ova and cysts although it only preserves the internal morphology of the cysts for up to 6 months, after which the cytoplasm of the organism becomes granular with poor nuclear definition.
Sodium Acetate Acetic Acid Formalin (SAF)
SAF fixed material is suitable for direct examination, concentration (Formalin/Ethyl Acetate) and permanent staining.
Merthiolate-Iodine Formalin (MIF)
Formol-Ethyl Acetate concentration methods can be performed on samples preserved in MIF.
Polyvinyl Alcohol (PVA)
This method will preserve ova, larvae and trophozoites well, but cysts may show some distortion. However, some ova and cysts do not concentrate well when preserved in PVA.
Parasitology Reagents
Mayer’s Glycerin-Albumin
Mayer’s Glycerin-Albumin is used when preparing slides for staining. The albumin helps to ensure that the specimen adheres to the slide and the glycerin retains sufficient moisture to prevent distortion or disruption of organisms on drying.
Triton X-100 Solution
Used to emulsify parasites in faeces, for use in standard Parasep® protocols.
Ethyl Acetate
A solvent that removes fat from faeces, for use in standard Parasep® protocols.
Acetone
Solvent
Neutral Red
Aqueous solution and dye, can be used in the Gram’s technique.
Parasitology Stains
Eosin/saline
This stain is useful for the detection of motile trophozoites of Entamoeba species.
Acridine orange (acetate buffered)
The addition of acridine orange to a faecal concentrate highlights the chromidial bars of Entamoeba coli, Entamoeba histolytica/ dispar and Entamoeba hartmanni, which fluoresce bright green.
Auramine phenol (lempert)
Method
1. Make faecal smears as for ZN and fix in methanol.
2. Stain with Auramine-Phenol (Lemperts) for 10 – 15 min.
3. Rinse thoroughly in tap water.
4. Decolourise in acid alcohol (as for ZN).
5. Rinse thoroughly in tap water.
6. Counterstain with 0.1% potassium permanganate for 30 sec.
7. Rinse thoroughly in tap water, allow to air dry. Do not blot dry, many brands of blotting paper will fluoresce!
Results in Oocysts appearing as bright yellow discs against a dark background.
Field’s Stain – Solution A and Solution B
N.B. Both solutions are ready for use and should not be diluted.
This technique is a rapid Field’s stain method, which enables rapid staining of fixed thin films of various clinical samples. This particular method is very useful for staining films of unformed faeces, faecal exudates, duodenal aspirates etc.
Method
1. Make a thin film of faeces/exudate and allow to dry.
2. Fix in methanol for 1 min.
3. Flood slide with 1 ml of Field’s Stain B .
4. Immediately add an equal volume of Field’s Stain A mix well on slide and allow to stain for 1 min.
5. Rinse well in tap water and drain dry.
6. Examine the film using the oil immersion objective and immersion oil.
Results
Parasite nuclei and structures containing chromatin – red
Cytoplasm – bluish-grey
Leucocyte nuclei – purple
Yeasts and bacteria – dark blue
Giemsa Stain Rapid
Giemsa stain can also be used to stain films of unformed faeces, faecal exudate, duodenal aspirates etc.
Lugol’s Iodine – Aqueous
Temporary stain for protozoa.
Iron Haematoxylin – Solution A and Solution B
Preparation Of Working Iron Haematoxylin Solution –
Method
1. Mix equal volumes of the two solutions and filter.
2. Allow to stand at least two hours (preferably overnight) before use in order that the chemical reaction is complete. Staining is optimum 3-5 days after preparation.
If the Iron Haematoxylin Solution is used immediately after preparation the parasites may be stained an intense blue with little nuclear detail differentiation.
When the Iron Haematoxylin stain is mature (usually 3-5 days after preparation) the background should stain grey with the protozoa light blue, and the nuclei blue-black. The background staining of the slide depends on the composition of the specimen.
The stain is normally useable for a week. Shelf life can be extended by storage in a stoppered bottle in the dark after each use.
Trichrome for Microsporidia
Method
1. Make smears from unconcentrated stool specimens in 10% formalin (1:3 ratio).
NOTE: ensure that the smears are extremely thin.
2. Fix in methanol for 5 mins.
3. Stain in modified Trichrome stain (code 1489) for 90 mins.
4. Rinse in acid alcohol for 10 secs.
5. Rinse briefly in 95% alcohol.
6. Place in 95% alcohol for 5 mins.
7. Place in 100% alcohol for 10 mins.
8. Clear in Xylene for 10 mins.
9. Examine under x 100 oil immersion objective, using Immersion Oil.
Interpretation
Microsporidial spores are ovoid and refractile and the spore wall stains bright pinkish-red. Occasionally the cellular content of some spores does not stain and appears transparent, others show a pinkish-red stained belt girding the spores either diagonally of equatorially.The spores are approximately 1.5 by 0.9µ. The background debris and bacteria are counterstained faint green.
Trichrome for Protozoa
May be used to stain fresh faeces, prefixed faeces (only certain fixatives) or cultured organisms. The method varies slightly depending on the sample preparation used
Malachite Green
Malachite Green is a green counterstain used to differentiate bacteria
Modified Z/N Stain Pack (Cold Kinyoun)
Suggested Method
1. Flood the heat fixed smears with Carbol Fuchsin (ZN) and steam gently for 5 minutes.
Add more stain if necessary to prevent drying.
2. Wash with water and decolourise with acid-alcohol until the dye no longer runs off the slide.
3. Wash with water and counterstain for 10-30 seconds with Methylene Blue or Malachite Green.
4. Wash, blot dry and examine.
Acid fast organisms stain red, the background and other organisms stain blue
Microbiology Stain Reagents
Gram Stain Protocol
Grams Iodine – Crystal Violet
Safranin O – Neutral Red
Gram’s Fuchsin – Gram’s Decolouriser
Gram’s Stain distinguishes between the two major classes of bacteria due to the differences in cell wall structure ;
Gram-positive bacteria, remain coloured after the staining procedure, and gram-negative bacteria, which do not retain dye.
In the staining technique, cells on a microscope slide are heat-fixed and stained with a basic dye, Crystal Violet, which stains all bacterial cells blue. Iodide solution is then added that allows the iodine to enter the cells and form a water-insoluble complex with the Crystal Violet dye. The preparation is then treated with a decolourise solvent, in which the iodine-crystal violet complex is soluble.
Following solvent treatment, only gram-positive cells remain stained, possibly because of their thick cell wall, which is not permeable to solvent. After the staining procedure, cells are treated with a Counterstain which may be Safranin O, Gram’s Fuchsin or Neutral Red. Counterstained gram-negative cells appear red, and gram-positive cells remain blue.
Although the cell walls of gram-negative and gram-positive bacteria are similar in chemical composition, the cell wall of gram-negative bacteria is a thin layer sandwiched between an outer lipid-containing cell envelope and the inner cell membrane, whereas the gram-positive cell wall is much thicker, lacks the cell envelope, and contains additional substances, such as teichoic acids, polymers composed of glycerol or ribitol.
The difference in reactivity between gram-positive and gram-negative bacteria is linked with differences in physiological properties of the two groups. Gram-positive bacteria are generally more sensitive to growth inhibition by dyes, halogens, many antibiotics, and to attack by phagocytosis and are more resistant to digestion by the enzymes pepsin and trypsin and enzymes in animal sera.
Gram Stain Pack
Contents
A Crystal Violet
B Grams Iodine Diluent
C Grams Iodine Concentrate
D Grams Decolouriser
E Counterstain: choice of (Safranin O, Neutral Red or Gram’s Fuchsin)
Note
The Grams Iodine Concentrate (C) should be added to the Diluent (B) and mixed well before use. All solutions are now ready to use in dropper bottles.
Lugol’s Iodine
The addition of iodine to a stool concentrate highlights the
internal inclusions of cysts; e.g. the nuclei and glycogen mass, thus aiding their identification.
For example, the addition of iodine enhances refraction of the nuclei of Endolimax nana, stains the peripheral chromatin of the nuclei of Entamoeba species and demonstrates the well-defined glycogen mass which is a feature of pre-cysts or immature cysts if E. coli and cysts of Iodamoeba butschlii.
Iodine does not stain the body of Entamoeba species.
Crystal Violet
Primary gram stain Crystal Violet solution is a violet stain used to differentiate bacteria.
Safranin O
Safranin O is a red stain used to differentiate bacteria, used in the Gram’s technique.
Gram’s Fuchsin
Used in the Gram’s technique
Lugol’s Iodine
Used in the Gram’s technique
Grams Decolouriser
Used in the Gram’s technique
